Pharmaceutical companies are rolling out “tamper-resistant” medicines- but how do their claims stack up to reality?
Feature image: Credit:Vera Kratochvil (PublicDomainPictures.net, available via CCL)
If you watch the news, chances are you hear some awful stories about illegal drugs. They are rampant, and with all the different kinds of addictive substances that are out there, it is no wonder that almost 6.8 million people in the U.S. have an addiction to illicit drugs.
Certain types of drugs are constantly covered in the media, such as heroin and other opioids, due to their high death rates. However, another drug has been on the rise: methamphetamine, or meth. The rates of abuse for this drug have remained quite high, but the rates of death involving meth and meth-like substances have nearly quadrupled from 2011 to 2017. In some parts of the country, mainly the Midwest and the West Coast, rates of abuse are higher and constantly leave law enforcement scrambling.
Meth is largely produced in “clandestine labs,” secretive labs used to produce a variety of illicit drugs. They can be found throughout the country and vary in size and scope of operation. Those running these labs tend to use commercially available products (like batteries and cough medicine) and equipment (such as large soda bottles) to make their products. As such, the government has placed regulations on most substances and products that can be used to make these drugs. One such product is pseudoephedrine, which is commonly found in decongestants and can be used as a pre-cursor to manufacture meth.

Figure 1. Police officer in a hazmat suite investigates a lab. Police have to wear hazmat suits to inspect clandestine labs, which often have highly unsafe conditions and toxic chemical waste. Credit: “GRC_5618” by DIVISION C – QUÉBEC (Flickr, available via CC BY-NC-ND 2.0 )
Some pharmaceutical companies have created new “tamper-resistant” formulas for their decongestants, claiming that these changes prevent the formulation of meth from this medicine. To confirm these claims, a team from the NMS toxicology labs and the Prescription Drug Research Center performed several assessments of two tamper-resistant pseudoephedrine medicines to determine how much these drugs would inhibit methamphetamine yield. They evaluated two brands (Zephrex-D® from Westport Pharmaceuticals. and Nexafed® from Acura Pharmaceuticals) against a pseudoephedrine drug that was not marketed as tamper-resistant (Sunmark® from Strategic Sourcing Services).
To maintain as much authenticity as possible for their study, Presley et. al. used real (readily available on the Internet) recipes for cooking meth and obtained as many commercially available equipment and products as possible. The method they followed is referred to as the “one pot” method, where all necessary reagents are mixed and cooked in a 2-L soda bottle.
The researchers analyzed two variations of the one-pot method: the direct and the indirect methods. The direct method used the whole, ground decongestant tablet in the cooking. The indirect method entailed separating the pseudoephedrine from the other tablet ingredients by dissolving the ground tablet in a water solution containing a strong base, mixing with an organic solvent, and boiling off the solvent to get solid pseudoephedrine. They analyzed their samples using liquid chromatography-tandem mass spectrometry (LC-MS/MS). This technique allowed them to determine how much pseudoephedrine was in their extracted samples and how much had been converted into meth product or recovered.
The team found that, regardless of the formula, all three decongestants resulted in some formation of methamphetamine. Under ideal conditions, the scientists determined that the original recipe converted 50-65% of pseudoephedrine into meth, while the rest of pseudoephedrine remained in solution. However, true to the claims of Westport and Acura Pharmaceuticals, the amount of meth made from their brands was significantly less than the non-tamper resistant Sunmark (on average, 49% less).
Between the two variations, the direct one-pot variation produced 93% more product than the indirect. In the direct variation, Zephrex-D yielded 90% less meth than Nexafed, whereas Zephrex-D yielded 68% more in the indirect. The researchers reported that less pseudoephedrine could be separated from Nexafed (31% less than Zephrex-D and 24% less than Sunmark), which would explain why it produced less meth in the indirect method.
| Formulation | Trial | Meth Production- Direct Method | Meth Production- Indirect Method |
| Zephrex-D | 1 | 17.3 | 9.3 |
| 2 | 12.4 | 11.9 | |
| Nexafed | 1 | 51.2 | 2.4 |
| 2 | 27.0 | 8.0 | |
| Sunmark | 1 | 62.4 | 50.1 |
| 2 | 60.8 | 2.2* |
Table 1. Methamphetamine production from the direct and indirect one-pot methods. *The researchers noted that the second trial for Sunmark under the indirect method did not proceed correctly.Credit: recreated from Presley et. al. J Pharm Biomed Anal, 2018.
Presley et. al. furthered their investigation by optimizing the original one-pot method. Clandestine drug makers are continuously optimizing their own recipes to increase their yield and could also do so to bypass the inhibitory effect of the tamper-resistant drugs. The researchers altered the method to see whether the tamper-resistant qualities of the Zephrex-D and Nexafed decongestants could stand against improved recipes. They expanded on the direct one-pot method, as this already produced more meth than the indirect variation.
The main parameter they altered was the length of the cooking step. The scientists used LC-MS/MS to analyze the mixture as it cooked about every 10 minutes for up to 3 hours. They found that the time for maximal meth yield was at about 150 minutes (2.5 hours). With the optimized cook time, the meth yield for all medicines increased greatly. For Sunmark, the meth conversion rate increased by 6%, which makes sense as the rate was already high before. For Zephrex-D, it increased by 103%, and for Nexafed, 42%. This indicates that changing the basic recipe would allow meth cookers to bypass the safety measures introduced by the pharmaceutical companies.
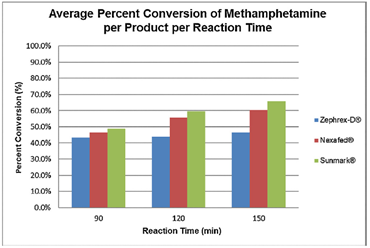
The initial implications of the research may appear bleak. Despite these companies’ attempts to hinder the illegal production of meth from their medicines, Presley et. al. showed how meth cookers would still be able to produce a decent amount of product from tamper-resistant medicines. However, not all is lost. While the Sunmark and Nexafed tablets still produced the normal maximum yield of meth amount under optimized conditions, the Zephrex-D consistently produced amounts of meth below this amount (about 46%) regardless of method variations. This suggests that the pharmaceutical companies are heading in the right direction and may soon be able to seriously or even completely thwart the production of destructive, illegal drugs.
| Title | Efficiency of extraction and conversion of pseudoephedrine to methamphetamine from tamper-resistant and non-tamper-resistant formulations |
| Author(s) | Brandon Presley, Bob Bianchi, John Coleman, Fran Diamond, Gerry McNally |
| Organization(s) | NMS Labs, PA; Prescription Drug Research Center, FL & VA; Johnson & Johnson Consumer, Inc., PA |
| Year | 2018 |
| Journal | Journal of Pharmaceutical and Biomedical Analysis |
| Link | https://www.sciencedirect.com/science/article/pii/S0731708517331904?via%3Dihub |

One thought on “Breaking the Bad: can medical companies prevent criminals from making drugs?”