Imagine you’re a fledgling crime scene investigator. You enter a house and find a small amount of white powder on a coffee table. The powder is spread-out, but could have been a straight line. On the coffee table is an empty donut box with some remaining powder inside. With just this information, can you determine if the powder is cocaine or powdered sugar (Fig 1)? What if the powder is a mixture? Will you be able to analyze the mixture in such a way to get a representative concentration of cocaine and powdered sugar molecules?

Such concerns for detection and identification are further magnified in drug mixtures, where your analytical system may not be set up to detect all the miscellaneous items present in street drugs. For example, street heroin samples often contain other illicit substances and fillers such as baby powder or baking soda, which can complicate analysis. How then to determine if your sample is innocent donut debris or a controlled substance, without wasting sample? The answer might surprise you.
A team from Harvard University’s chemistry department and the Drug Enforcement Administration unveiled an unusual and elegant solution to this problem in December 2019—magnetic levitation.

Instead of using sample-consuming methods to separate and then identify the compounds in a mixture (like what is done in chromatography coupled with mass spectrometry methods), the authors proposed that separating a powder’s components before analysis could allow an initial pre-screening of different compounds. They believe that sorting powders into categories by their densities, and then analyzing these purified samples greatly increases their chance of chemical identification (Fig 2).
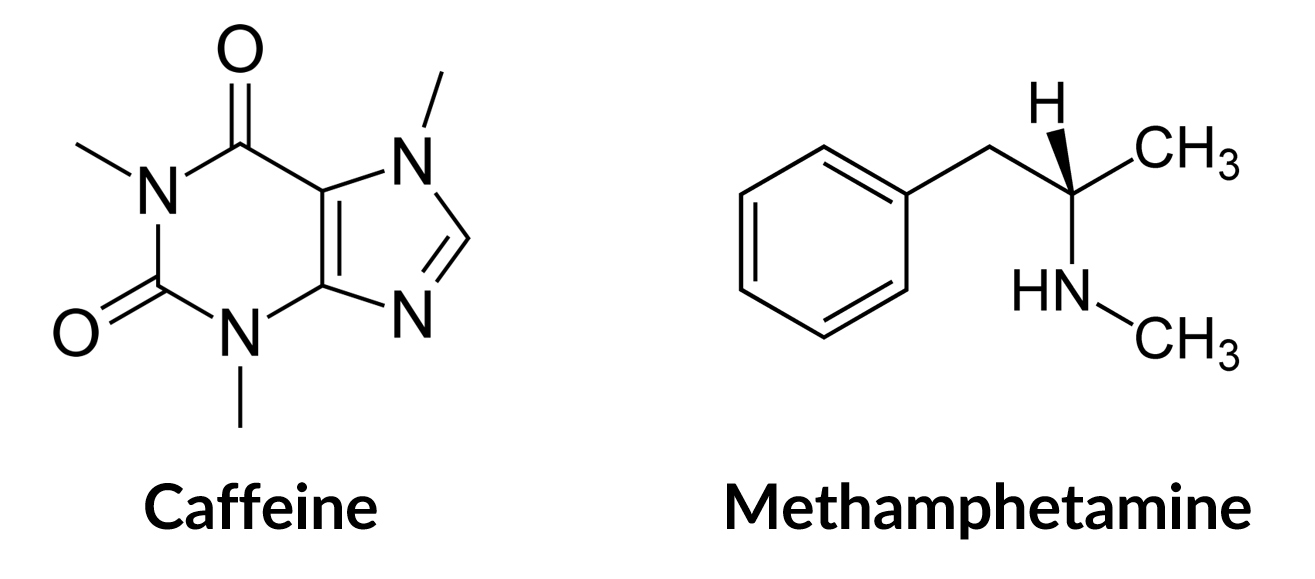
Compounds have different densities dependent upon their mass per volume. Molecules of varying sizes, represented by their chemical structure, will fill the same volume (or space) differently. For example, a caffeine molecule will fill more of the same space than a molecule with smaller or more flexible chemical structure, such as methamphetamine (Fig 3).
In the magnetic levitation, or MagLev, system, substances with larger densities will sink to the bottom while substances with smaller densities will rise to the top of the cuvette. So, in a powder mixture of caffeine and methamphetamine, caffeine would sink, while methamphetamine would rise. The key in this method is to not use a liquid that will dissolve the compounds themselves—the powders must be suspended (like oil droplets in water) to separate properly.
There are many advantages to this system. Once separated, police officers and other non-expert personnel can visually compare the height of each compound to a known sample list and immediately detect illicit drugs. At the same time, they can get a rough idea of how much illicit drug is present in comparison to other components.
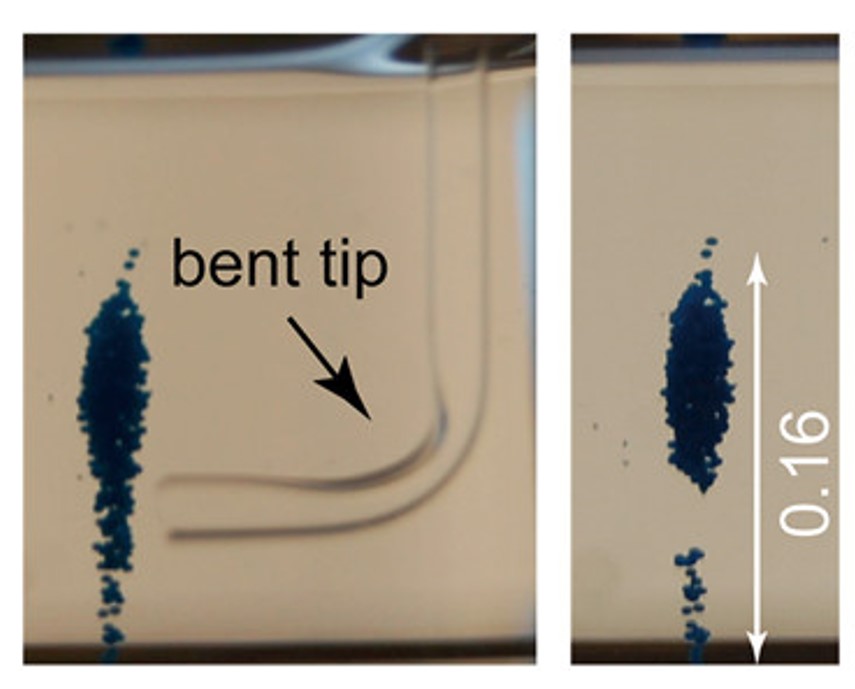
The researchers demonstrated this proof of concept by magnetically levitating pure standards, and then separating an artificial mixture in the lab. Within 5 minutes, a mixture of fentanyl, heroin, methamphetamine, cocaine and a fentanyl-analogue separated into its individual components with little overlap. Remarkably, in a mixture containing 99% caffeine and 1% lidocaine, the magnetic system completely separated the lidocaine component in under 20 minutes. The separated powders could then be removed using a bent cuvette (Fig 4) and analyzed with standard confirmatory practices—this resulted in readouts that looked almost identical to pure standards!
Abrahammson et al.’s MagLev system is portable, rapid, easy to use— and even aesthetically appealing. Once the authors (or others) apply this technology to casework samples, validate their efficacy compared to current methods and partner with a company to develop a prototype, MagLev could be in the field as early as 2025. Fingers crossed me and other forensic students get to do some levitating out in the field!
| Title | Analysis of Powders Containing Illicit Drugs Using Magnetic Levitation |
| Authors | Christoffer K. Abrahamsson, Amit Nagarkar, Michael J. Fink, Daniel J. Preston, Shencheng Ge, Joseph S. Bozenko Jr., George M. Whitesides |
| Journal | Angewandte Chemie |
| Year | 2019 |
| Link | https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201910177 |
