When you hear the word “fireworks,” what are you typically reminded of? Parades, celebrations, happy memories? These marvels fill the air and light up the night sky with dazzling displays of colors and sounds. However, forensic scientists see these as an opportunity to reveal and investigate underlying problems within their respected communities.
In the Netherlands, illegal fireworks known as “flash bangers,” cause trouble for the police. These items are often more powerful than traditional fireworks because of the composition of the powders. Most of the incidents reported are property damage (Figure 1) or personal injuries. However, there are criminal reports of the destruction of bank ATMs, deaths, and and even manufacturing improvised explosive devices (IEDs).

Currently, forensic analysts focus on features of pre- or post-explosive devices. A team led by Dr. Adrian van Asten from the University of Amsterdam chose to push the boundaries of identification and explore possible chemical trends of the powders. The main compounds under investigation are inorganic (meaning non-carbon based or metal containing) mixtures such as ammonium nitrate and potassium perchlorates, as well as certain metals. These are commonly found in “factory-made” fireworks. However, if fireworks are made in clandestine or illegal areas, they can possess impurities consistent with that area. This allows investigators to potentially localize the area of production. With those impurities, scientists can generate databases like the University of Central Florida’s smokeless powder or ignitable liquids database, sharing information obtained in an academic setting for investigative use.
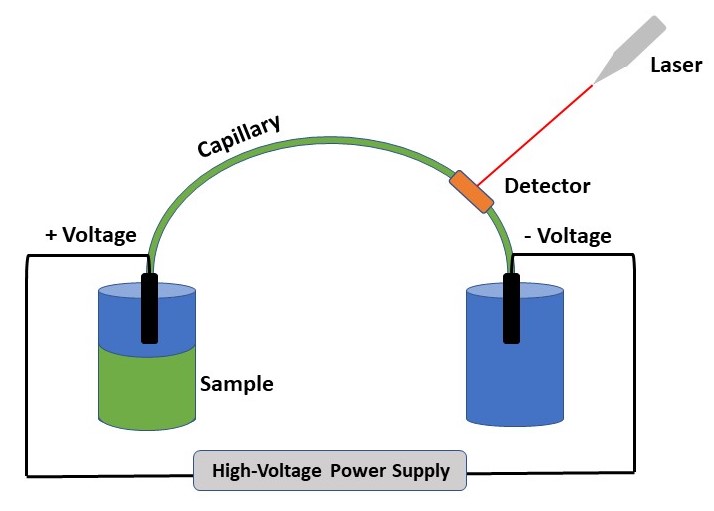
The researchers investigated these impurities using using capillary electrophoresis (CE) for separation with indirect ultraviolet (UV) detection to potentially yield a more objective identification (Figure 2). Unlike direct UV detection, where the laser excites the analyte of interest and a detector monitors the resulting light emission, indirect UV detection causes an interactions between analytes of interest with ions in solution. This interaction is what produces the detected light emission, and how the compounds are identified. CE with indirect UV detection allows for quick separation (< 10 minutes) of the components by applying specific solvents and voltages to a thin glass tube.
Three sub-types of high-powdered flash bangers known as “Cobra 6” were obtained from the Dutch police department and disassembled to access the stored powder. The sub-types included the commercially available Cobra 6 and Cobra 6 2G, and an imitation Cobra 6 flash bangers.
These powders were observed under a microscope for physical differences and could not be distinguished, necessitating the analysis of chemical characteristics to provide more information. The authors explored the intact powder composition and found that potassium nitrate (common explosives enhancement; abbrev. KNO3) and potassium perchlorate (coloring in fireworks; abbrev. KClO4) were the main compounds for both black powder and imitation mixes shown in Figure 3.

After visual screening, the authors used solvents to extract and isolate compounds from their confiscated samples. In addition to the main components, flash bangers may have impurities present such as calcium or magnesium originating from either the production environment or to enhance the explosive potential. They observed that the “Cobra 6” brands had no additional additives present. Conversely, the imitation mix had a high concentration of magnesium and calcium impurities. It is important to note that calcium may not be a reliable indicator because it can found in black and flash powder mixtures to improve the colors of fireworks.

The next steps included grouping the chemical signatures (signal intensities from UV) and comparing them, illustrated by Figures 3, 4, and 5. Samples from different locations were identified and categorized into the Cobra 6 imitation set or commercial sets by comparing different analytes at the same time. This is achieved by using principal components analysis (PCA), a statistical method that groups samples based on their similarities. Essentially, the authors tested the composition of a sample by the weight percentage of perchlorates (ClO4-), magnesium, potassium (K+), and calcium (Ca2+) and compared them against others. For Figures 3 and 4, two analytes (K+ and ClO4-) and (Ca2+ and K+) were investigated to achieve some distinction between flash banger samples. Despite the limited sample size, the researchers could determine a difference between the imitation powders and the Cobra 6 powders. Conversely, there was difficulty in classifying the commercial flash bangers, Cobra 6 and Cobra 2G.
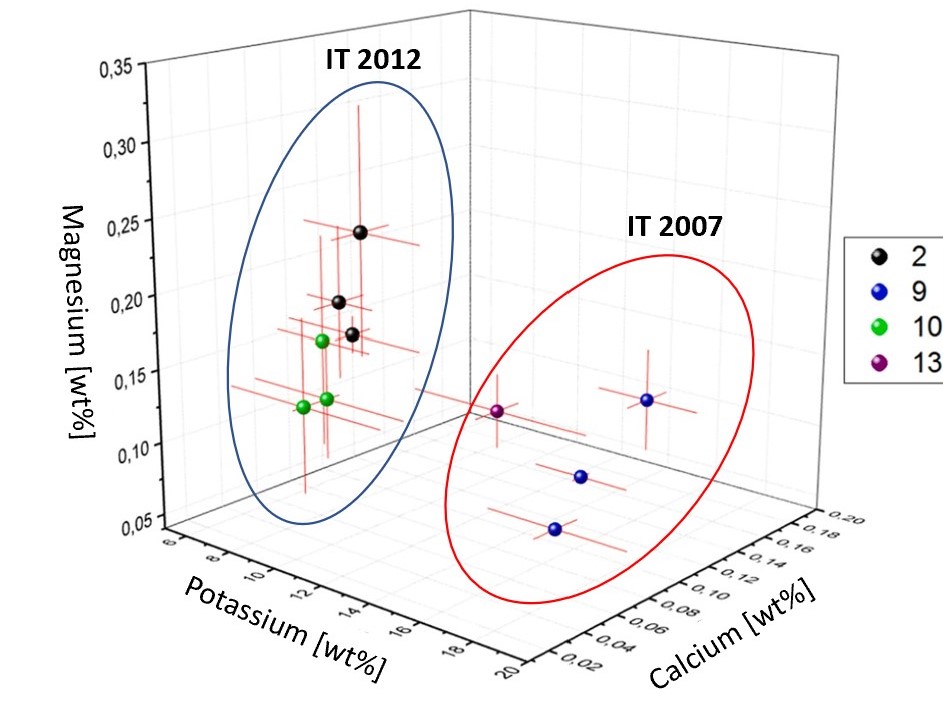
However, when the authors investigated three analytes simultaneously (magnesium, potassium, and calcium) using PCA, the Italian-made (IT) samples from 2007 (9 and 13) and 2012 (2 and 10) could be clearly identified as distinct and grouped separately (Figure 5).
This study using CE-UV detection provides the crucial steps to develop a quick, efficient classification methods for flash bangers. Future experiments can optimize this database using a more extensive sample set and a wider range of inorganic compounds. Completing this database will provide forensic scientists and police officers with another tool to act rapidly and proficiently in apprehending criminals.
| Title | Rapid forensic chemical classification of confiscated flash banger fireworks using capillary electrophoresis |
| Authors | Karlijn D.B. Bezemer, Lara V.A. van Duin, Carlos Martín-Alberca, Govert W. Somsen, Peter J. Schoenmakers, Rob Haselberg, Arian C. van Asten |
| Journal | Forensic Chemistry |
| Publisher | Elsevier |
| Year | 2019 |
| Link | https://www.sciencedirect.com/science/article/pii/S2468170919300840 |

One thought on “Fireworks provide insights to dangerous homemade explosives”