A team in Spain developed a rapid and accurate way to detect chemical warfare agents- in the air.
Feature Image: Credit: “Person in black gas mask”(Pikrepo via CC0)
For most of us, chemical warfare seems to be a thing of the past. The advent of chemical warfare agents (CWAs) came in World War I, as forces on both sides used tear and mustard gas on each other. Since then, more advanced and deadly forms of CWAs have been developed and found in the United States as recently as 2019. In January, the FBI found phosgene, a deadly choking gas, in an Oklahoma home. CWA’s can have lifelong consequences on those exposed, both physically and mentally. Nerve agents like sarin and VX break down important neurochemicals used to transmit information between nerve cells, causing paralysis and eventually death. Nerve agents are highly toxic, and often even a miniscule amount can be fatal. Sarin has a lethal dosage of 1 mg per 1 m3 of air, which is equivalent to 1 pound of chemical in 120 million gallons of water. Given the harm that CWA’s can do, police need a means to constantly monitor for them, especially in large public spaces or events.

Many research groups have used Raman spectroscopy to detect and analyze different forms of evidence, such as blood and drugs. Raman spectroscopy uses laser light to irradiate, or shine on, a sample, which then scatters this light based upon its chemical composition, and in a way that is unique to the sample. The profile of all these signals from a sample creates a spectrum, which will also be unique to a sample. This allows Raman to distinguish between different chemicals and would make it highly effective at detecting a CWA amongst all other matter present in air. A specific type of Raman spectroscopy, known as Surface Enhanced Raman Spectroscopy (SERS), equips a testing surface (or substrate), usually made of metals like gold or silver, to enhance the signal of a sample. This allows for the analysis of very small specimens, such as air particles (Figure 2).
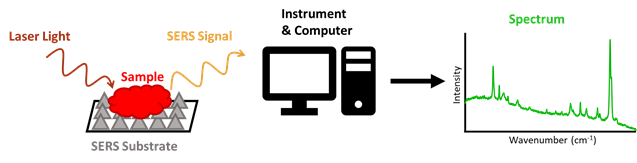
Led by Dr. Reyes Mallada of the Nanostructured Films and Particles group at Aragon Nanoscience Institute, PhD student Marta Lafuente evaluated if SERS could be used to detect nerve agents in air. According to these researchers, most other studies using SERS to detect toxic chemicals have only done so for chemicals in a liquid phase, or have only detected their gas forms at concentrations much higher than their lethal doses. The few studies that were able to detect very low concentrations of toxins in the air were able to do so by selecting SERS substrates that had strong interactions with the toxin, such as gold and silver to detect NO2. These interactions are crucial, because the substrate can only enhance the signal of particle it is in contact with.
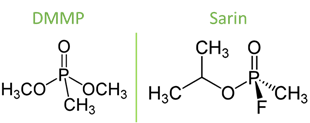
As such, Lafuente et. al. compared three different types of surfaces to determine which would be the best at detecting a low concentration of DMMP (14 mg/m3). Dimethyl methylphosphonate (DMMP) is a chemical with a similar structure to sarin but much less harmful, and therefore used in research to simulate sarin (Figure 3). They chose silver as a base for the substrates, because silver is known to have a specific interaction with DMMP through its phosphate-oxygen (P-O) bond, which would make enhancement of the chemical’s signal more intense. The silver was then paired with a support, either a silicon wafer, a stainless-steel mesh, or a graphite foil. The surfaces were housed in a metal box, where streams of air could be passed through using a tube. A small, portable Raman spectrometer was used to analyze this gas, through a small opening at the top of the box.
The researchers used three different laser wavelengths to irradiate the samples, to determine if a certain laser would allow for a clearer signal over the others. The wavelengths they used were 532 nm, 633 nm, and 785 nm. For this step, they analyzed another chemical (rhodamine-6-G) that gives clear, defined peaks in its Raman spectra. Lafuente’s et. al. wanted a wavelength-substrate combination that would cause the least amount of fluorescence, another type of light phenomenon that can cause a background (miscellaneous) signal that can overpower the Raman signal of the chemical of interest. To determine the best combination, the scientists calculated the “Analytical Enhancement Factor” (AEF), which dictates the difference in a signal between normal Raman and SERS analyses. The larger the AEF, the greater the difference and the more enhanced the signal became when using SERS. The group concluded that the 785 nm laser had the greatest AEF when used in combination with the silicon and graphite-supported surfaces.
When testing gaseous DMMP with these lasers and substrates, Lafuente et. al. found the strongest signal response from the 785 nm laser with the graphite substrate. The spectrum of gaseous DMMP obtained was very similar to that of liquid DMMP, indicating that the spectrum belonged to DMMP and not another particle found in the air. The DMMP spectra has a relative standard deviation of 5%. This meant that the technique used in the experiment was very reliable, and that the spectra collected using it would be highly reproducible. This is important in forensics, where multiple tests might have to be run on a piece of evidence to confirm previous results. Furthermore, the researchers determined that a quality spectrum of DMMP gas could be collected in only 137 seconds!

The work of Lafuente and her colleagues demonstrated that portable SERS could rapidly and reliably detect very small traces of toxic nerve gases in the air, namely when using a graphite-silver substrate and a 785 nm laser. This offers a major advancement in detecting CWAs in their gas forms. Given that the instrument used is small and portable, and has a very short analysis time, police can use this technique to analyze the air at frequent intervals to quickly determine the presence of a toxin in the air. The length of exposure to CWAs correlates with the intensity of their effects and their fatality. As such, giving the police a way to rapidly detect CWAs will allow them to save numerous lives.
Citation:
| Title | Gas phase detection of chemical warfare agents CWAs with portable Raman |
| Author(s) | Marta Lafuente, Diego Sanz, Miguel Urbiztondo, Jesús Santamaría, María Pilar Pina, Reyes Mallada |
| Organization(s) | Nanoscience Institute of Aragon, Instituto de Ciencia de Materiales de Aragón, and Centro Universitario de la Defensa de Zaragoza (Zaragoza, Spain) Networking Research Center on Bioengineering, Biomaterials and Nanomedicine (Marid, Spain) |
| Year | 2020 |
| Journal | Journal of Hazardous Materials |
| Link | https://www.sciencedirect.com/science/article/abs/pii/S0304389419312336?via%3Dihub |

One thought on “Can Raman spectroscopy protect us from nerve gas?”