Image for feature photo from Pexels
Unlike in other scientific fields, motivation for forensic science research often originates from a legal standpoint. One such example is determining the exact mechanism of death, including indirect contributors. Recently, a collaborative case study between two Romanian universities revealed that hydrogen cyanide, which is known to cause cyanide poisoning via smoke inhalation in enclosed spaces, may also contribute to death via open-space fires. Case studies connect the realities practitioners encounter in the field with scientific rigor to answer a specific question or illustrate a problem. Researchers at the Transylvanian University of Brasov and University of Iasi in Romania used the case study of an 80-year-old man to investigate hydrogen cyanide’s contribution to death during an open-space fire.
Fire-related deaths can be a result of an accident, natural causes (like death by heart attack before the fire) or homicide; distinguishing between these manners of death is dependent upon the specific injury that caused the fatality. Nowadays, it is known that most fire-related deaths are caused by inhaling the toxic chemicals in smoke, such as carbon monoxide (CO). This tiny molecule binds to the proteins in your red blood cells and tissues and prevents them from carrying oxygen, thereby suffocating your body. However, smoke also contains other toxic molecules that arise from the burning of different materials. For example, burning clothing or plastics produces hydrogen cyanide, another tiny molecule that is 25 times more toxic than CO. Hydrogen cyanide saturates the blood within 10 minutes of exposure and prevents your body from generating energy, halting your metabolism entirely.

In the firefighting industry, these two chemicals combined are known as the “toxic twins” and have been a well-documented concern. But when it comes to the science of death, the literature is conflicting. Most toxicological analysis on fire-related deaths looks for carbon monoxide poisoning greater than 50% in the blood, which is considered lethal and therefore the major cause of death. If less is found, then the victim was potentially dead before the fire, and therefore more investigation as to the cause and manner of death are required. Cyanide poisoning via hydrogen cyanide, on the other hand, while acknowledged as a death contributor in enclosed fires, is not routinely included in toxicological analysis. It’s not that traditional methods suffer from accuracy issues; there is no information on how quickly hydrogen cyanide disappears from the blood and measurements made days or even hours postmortem show vastly different results.
Would an 80-year-old man burning trash in an open field be exposed to high levels of hydrogen cyanide and justify inclusion of cyanide analysis in routine forensic workups? That’s what the authors Tabian and colleagues set out to discover. But before they could proceed, they had to develop a method to determine hydrogen cyanide levels postmortem. To combat their dissipating killer, Tabian et al. used a method to convert hydrogen cyanide to a more stable product using a common forensic tool – ninhydrin.
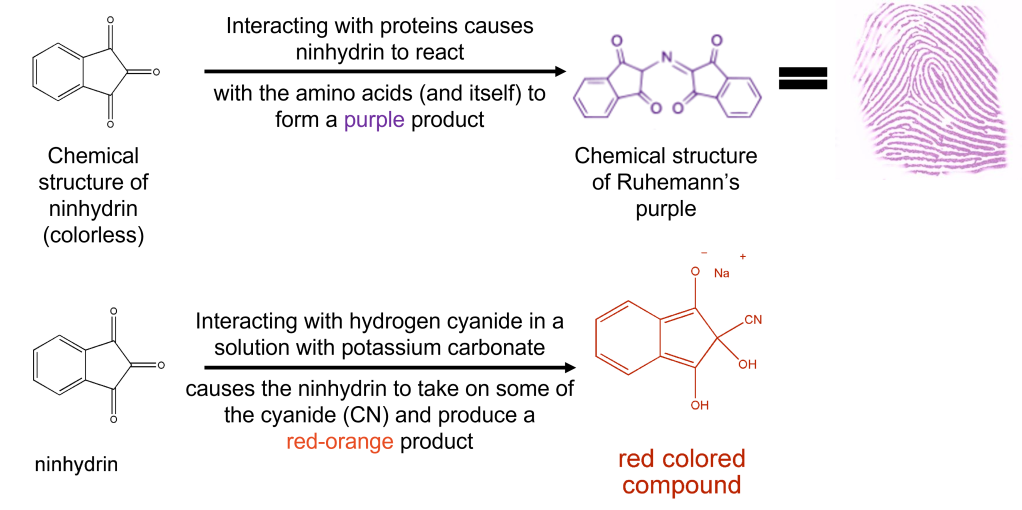
Forensic scientists commonly use ninhydrin to make invisible fingerprints appear by reacting with the proteins deposited from your finger (Fig 2 top reaction). However, Tabian and colleagues found a method used to determine the amount of cyanogen (a closely related compound of hydrogen cyanide) in plants with ninhydrin and applied it to their problem of disappearing cyanide in human blood. When hydrogen cyanide reacts with ninhydrin, the cyanide molecule attaches itself to ninhydrin, producing a stable red-orange product monitored with a UV-Vis spectrometer (Fig 2 bottom reaction). After performing toxicological analysis on the victim’s blood, they found cyanide levels at 1.3 ug/ml – half that of the completely lethal dose level of 3 ug/ml. Mixed with the 73% CO level in the victim’s blood, the hydrogen cyanide becomes much more potent.
The question then became where did the hydrogen cyanide originate? Since wood and most trash do not produce hydrogen cyanide, the authors hypothesized that the man’s clothing, or potentially plastic bottles, which had been set alight by the blaze were the source. The authors concluded that the hydrogen cyanide in the smoke incapacitated the victim and prevented him from putting out the fire that spread out of control from his trash pile. The toxic chemicals from smoke inhalation were the cause of death, and the manner of death was ruled accidental.
Tabian et al.’s careful investigation of the case study of an elderly Romanian man opens up the conversion on compounding factors during a fire-related death that significantly contribute to fatalities. While cyanide poisoning is debated in the literature as a consistent, major cause for fire-related deaths, especially in open spaces, the high levels of hydrogen cyanide in the victim’s blood suggest greater consideration of the toxic molecule during the investigative process. This can not only lead to further information for crime scene recreation but also more careful commercial practices and product development.
| Title | Hydrogen cyanide and carboxyhemoglobin assessment in an open space fire-related fatality |
| Authors | Daniel Tabian, Diana Bulgaru Iliescu, Tatiana Iov, Barabas Barna, Sebastian Ionut Toma, Gabi Drochioiu |
| Journal | Journal of Forensic Sciences |
| Year | 2021 |
| Link | https://onlinelibrary.wiley.com/doi/10.1111/1556-4029.14649 |
